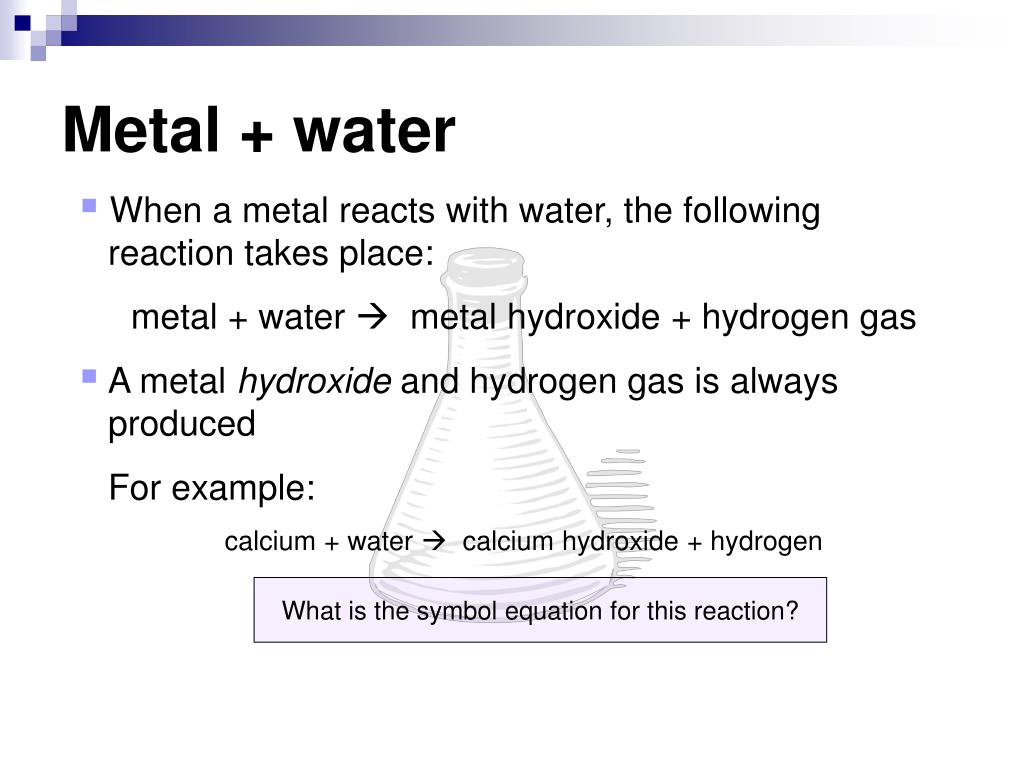
Metal React With Water Example . When a metal reacts with water, a metal hydroxide and hydrogen are formed. metals that react with water produce a metal hydroxide and hydrogen gas: Some react slowly, some react readily and violently. Metal + water → metal hydroxide + hydrogen gas. Potassium reacts quickly with water. metals react with water in a variety of ways. calcium, strontium, and barium. when metals react with water, metal hydroxides and hydrogen gas are formed. When a metal reacts with water, a. reactions of metals with water. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides.
from www.slideserve.com
reactions of metals with water. Some react slowly, some react readily and violently. calcium, strontium, and barium. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. when metals react with water, metal hydroxides and hydrogen gas are formed. Potassium reacts quickly with water. metals that react with water produce a metal hydroxide and hydrogen gas: all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Metal + water → metal hydroxide + hydrogen gas. When a metal reacts with water, a metal hydroxide and hydrogen are formed.
PPT Reactions of Metals PowerPoint Presentation, free download ID4271268
Metal React With Water Example as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. Some react slowly, some react readily and violently. calcium, strontium, and barium. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. When a metal reacts with water, a. Metal + water → metal hydroxide + hydrogen gas. reactions of metals with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. metals react with water in a variety of ways. Potassium reacts quickly with water. metals that react with water produce a metal hydroxide and hydrogen gas: when metals react with water, metal hydroxides and hydrogen gas are formed.
From www.youtube.com
Reaction of sodium metal with water YouTube Metal React With Water Example When a metal reacts with water, a metal hydroxide and hydrogen are formed. When a metal reacts with water, a. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. reactions of metals with water. Metal + water → metal hydroxide + hydrogen gas. Some react slowly, some react readily and. Metal React With Water Example.
From exoypnorc.blob.core.windows.net
Metal In Water Reaction at Glenn King blog Metal React With Water Example metals react with water in a variety of ways. reactions of metals with water. calcium, strontium, and barium. when metals react with water, metal hydroxides and hydrogen gas are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. These metals react with cold water with increasing. Metal React With Water Example.
From www.youtube.com
Reactions of Metallic Oxides with Water Chemistry Class 10 Embibe Achieve Karnataka Board Metal React With Water Example reactions of metals with water. Some react slowly, some react readily and violently. Potassium reacts quickly with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. When a metal reacts with water, a. metals that react with water produce a metal hydroxide and hydrogen gas: when metals react with water, metal hydroxides. Metal React With Water Example.
From ar.inspiredpencil.com
Metal And Water Reaction Metal React With Water Example when metals react with water, metal hydroxides and hydrogen gas are formed. metals react with water in a variety of ways. reactions of metals with water. metals that react with water produce a metal hydroxide and hydrogen gas: Potassium reacts quickly with water. as mentioned earlier, many group 1 and group 2 oxides react with. Metal React With Water Example.
From ppt-online.org
Metals презентация онлайн Metal React With Water Example metals react with water in a variety of ways. Potassium reacts quickly with water. When a metal reacts with water, a. metals that react with water produce a metal hydroxide and hydrogen gas: when metals react with water, metal hydroxides and hydrogen gas are formed. When a metal reacts with water, a metal hydroxide and hydrogen are. Metal React With Water Example.
From www.youtube.com
Sodium metal reaction with water YouTube Metal React With Water Example Some react slowly, some react readily and violently. When a metal reacts with water, a. metals react with water in a variety of ways. Potassium reacts quickly with water. Metal + water → metal hydroxide + hydrogen gas. calcium, strontium, and barium. as mentioned earlier, many group 1 and group 2 oxides react with water to form. Metal React With Water Example.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID4271268 Metal React With Water Example Some react slowly, some react readily and violently. When a metal reacts with water, a metal hydroxide and hydrogen are formed. When a metal reacts with water, a. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. as mentioned earlier, many group 1 and group 2 oxides react with water to form. Metal React With Water Example.
From www.slideserve.com
PPT Reactivity Series of Metals PowerPoint Presentation ID468914 Metal React With Water Example These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. Metal + water → metal hydroxide + hydrogen gas. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. when metals react with water, metal hydroxides and hydrogen gas are formed. When a metal reacts. Metal React With Water Example.
From www.tes.com
Lesson Alkali Metals GCSE Edexcel 91 by chemistryteacher001 Teaching Resources Tes Metal React With Water Example as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. metals that react with water produce a metal hydroxide and hydrogen gas: When a metal reacts with water, a. Potassium reacts quickly with water. reactions of metals with water. Metal + water → metal hydroxide + hydrogen gas. calcium,. Metal React With Water Example.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID4271268 Metal React With Water Example when metals react with water, metal hydroxides and hydrogen gas are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. calcium, strontium, and barium. When a metal reacts with water, a metal hydroxide and hydrogen are formed. When a metal reacts with water, a. Some react slowly, some. Metal React With Water Example.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID5525228 Metal React With Water Example all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. metals react with water in a variety of ways. Metal + water → metal hydroxide + hydrogen gas. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. reactions of metals with. Metal React With Water Example.
From www.slideshare.net
Metals Metal React With Water Example Metal + water → metal hydroxide + hydrogen gas. Some react slowly, some react readily and violently. When a metal reacts with water, a metal hydroxide and hydrogen are formed. when metals react with water, metal hydroxides and hydrogen gas are formed. calcium, strontium, and barium. metals react with water in a variety of ways. as. Metal React With Water Example.
From www.meritnation.com
Reaction of metals with water explain with examples the whole concept Science Metals and Non Metal React With Water Example When a metal reacts with water, a. Metal + water → metal hydroxide + hydrogen gas. When a metal reacts with water, a metal hydroxide and hydrogen are formed. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously. Metal React With Water Example.
From ar.inspiredpencil.com
Metal And Water Reaction Metal React With Water Example Potassium reacts quickly with water. calcium, strontium, and barium. reactions of metals with water. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Some react slowly, some react readily and violently. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. metals. Metal React With Water Example.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Home Revise YouTube Metal React With Water Example Potassium reacts quickly with water. Some react slowly, some react readily and violently. metals react with water in a variety of ways. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. metals that react with water produce a metal hydroxide and hydrogen gas: calcium, strontium, and barium. . Metal React With Water Example.
From ar.inspiredpencil.com
Metal And Water Reaction Metal React With Water Example reactions of metals with water. when metals react with water, metal hydroxides and hydrogen gas are formed. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. Metal + water → metal hydroxide + hydrogen gas. These metals react with cold water with increasing vigor to give the metal hydroxide. Metal React With Water Example.
From www.youtube.com
Reaction of Water with Aluminium Chloride YouTube Metal React With Water Example Potassium reacts quickly with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. metals that react with water produce a metal hydroxide and hydrogen gas: Some react slowly, some react readily and violently. metals react with water in a variety of ways. reactions of metals with water. These metals react with cold. Metal React With Water Example.
From www.pinterest.com
Alkali metals react with water to produce hydroxides. Alkali metal, Gcse chemistry, Chemistry Metal React With Water Example When a metal reacts with water, a metal hydroxide and hydrogen are formed. Potassium reacts quickly with water. Metal + water → metal hydroxide + hydrogen gas. calcium, strontium, and barium. These metals react with cold water with increasing vigor to give the metal hydroxide and hydrogen. when metals react with water, metal hydroxides and hydrogen gas are. Metal React With Water Example.